Quantum Dot Technology

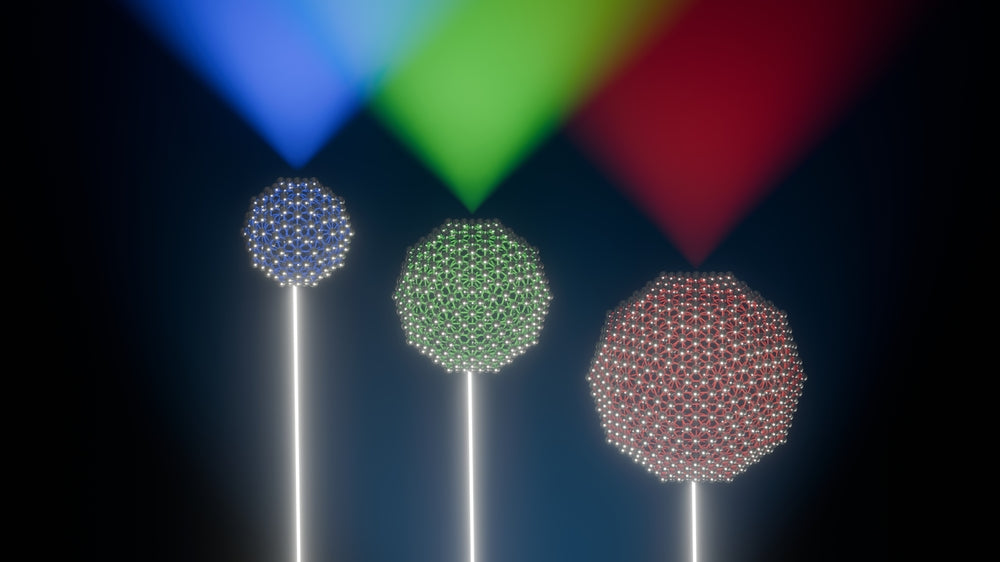
Quantum Dots (QDs) are nanoscale particles composed of II-VI or II-V elements, including atoms like zinc, cadmium, selenium, and sulfur. Their sizes generally range between 1 to 10 nanometers. Due to the quantum confinement of electrons and holes, the quantum confinement effect is particularly significant. This results in the transformation of a continuous energy band structure into a discrete energy level structure with molecular characteristics. Therefore, when Quantum Dots are excited, they emit extremely narrow peaks with high spectral purity. The emission spectrum of Quantum Dots can be controlled by altering their size and chemical composition, allowing their emission spectrum to cover the entire visible light range.
As a result, QD technology is applied in liquid crystal displays (LCDs). They serve as the excitation targets for blue LEDs, emitting purer red and green light under the excitation of the blue spectrum, thus expanding the color gamut of the display. QD technology is utilized in the backlighting of LCDs, primarily achieved through Quantum Dot Enhancement Films (QDEF), Quantum Dot Tubes (QD Tubes), or by encapsulating Quantum Dots in the LED lamp caps (QLED).
Since the advent of Quantum Dot technology, flat-panel displays have seen a revolution in high color gamut image quality, with NTSC color gamut coverage exceeding 100%. The vividness of the display quality can even rival that of OLED displays. However, as an emerging technology, the reliability of QD materials still needs improvement. Additionally, QD materials may contain cadmium, a heavy metal harmful to the environment. The moisture and high-temperature resistance of QD materials are poor. When exposed to moisture or high temperatures, the stimulated emission characteristics of QDs deteriorate, meaning their ability to emit red and blue light diminishes, which can cause the display to exhibit a bluish color shift.
